Indication Aimovig® (erenumab-aooe) is indicated for the preventive treatment of migraine in adults.
Indication Aimovig® (erenumab-aooe) is indicated for the preventive treatment of migraine in adults.
One dose, once a month, designed to keep working 24/7 to help reduce monthly migraine days1,2
Aimovig® offers the flexibility of 2 different dosing options1

In a usability study of the SureClick® autoinjector among
adult migraine patients (N = 204)4,†:
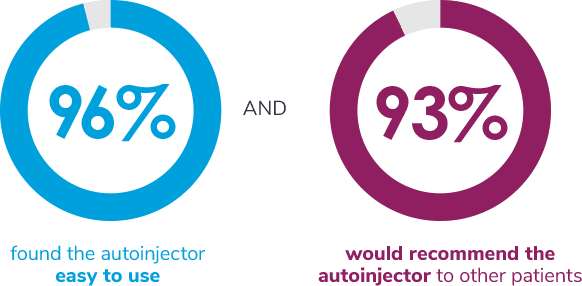
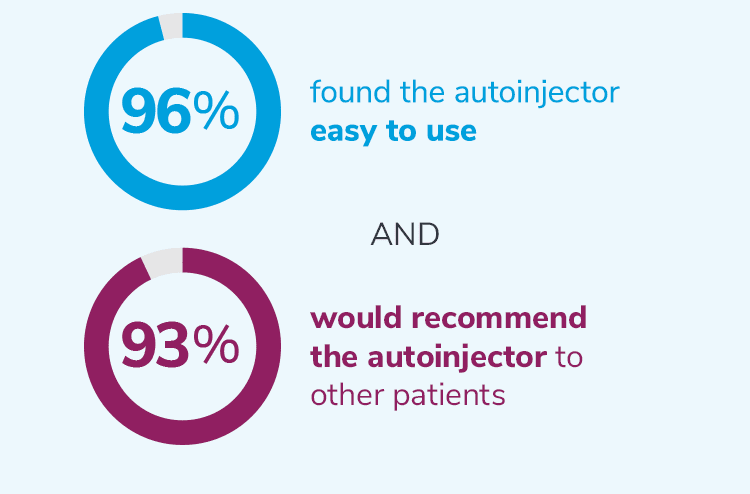
†204 autoinjector-naïve adult migraine patients were evaluated in a usability study of the Aimovig SureClick® single-dose autoinjector by performing a simulated injection. Percentage was derived from a combination of patients who stated completely agree or somewhat agree on a 5-point Likert scale.4
The AHS recommends assessing the benefits of anti-CGRP pathway mAb therapy after 3 months of treatment for those administered monthly.5
Show your patients this overview video on injecting Aimovig®
AHS = American Headache Society; CGRP = calcitonin gene-related peptide; mAb = monoclonal antibody.
Aimovig® (erenumab-aooe) is indicated for the preventive treatment of migraine in adults.
Contraindication: Aimovig® is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. Reactions have included anaphylaxis and angioedema.
Hypersensitivity Reactions: Hypersensitivity reactions, including rash, angioedema, and anaphylaxis, have been reported with Aimovig® in post marketing experience. Most reactions were not serious and occurred within hours of administration, although some occurred more than one week after administration. If a serious or severe reaction occurs, discontinue Aimovig® and initiate appropriate therapy.
Constipation with Serious Complications: Constipation with serious complications has been reported following the use of Aimovig® in the postmarketing setting. There were cases that required hospitalization, including cases where surgery was necessary. The onset of constipation was reported after the first dose in a majority of these cases, but patients also reported later on in treatment. Aimovig® was discontinued in most reported cases. Constipation was one of the most common (up to 3%) adverse reactions reported in clinical studies.
Monitor patients treated with Aimovig® for severe constipation and manage as clinically appropriate. Concurrent use of medications associated with decreased gastrointestinal motility may increase the risk for more severe constipation and the potential for constipation-related complications.
Hypertension: Development of hypertension and worsening of pre-existing hypertension have been reported following the use of Aimovig® in the postmarketing setting. Many of the patients had pre-existing hypertension or risk factors for hypertension. There were cases requiring pharmacological treatment and, in some cases, hospitalization. Hypertension may occur at any time during treatment but was most frequently reported within seven days of dose administration. In the majority of the cases, the onset or worsening of hypertension was reported after the first dose. Aimovig® was discontinued in many of the reported cases.
Monitor patients treated with Aimovig® for new-onset hypertension, or worsening of pre-existing hypertension, and consider whether discontinuation of Aimovig® is warranted if evaluation fails to establish an alternative etiology.
Raynaud's Phenomenon: Development of Raynaud’s phenomenon and recurrence or worsening of preexisting Raynaud’s phenomenon have been reported in the postmarketing setting following the use of CGRP antagonists, including AIMOVIG. Many of the cases reported serious outcomes, including hospitalizations and disability, generally related to debilitating pain.
AIMOVIG should be discontinued if signs or symptoms of Raynaud’s phenomenon develop, and patients should be evaluated by a healthcare provider if symptoms do not resolve. Patients with a history of Raynaud’s phenomenon should be monitored for, and informed about the possibility of, worsening or recurrence of signs and symptoms.
Adverse Reactions: The most common adverse reactions in clinical studies (≥ 3% of Aimovig®-treated patients and more often than placebo) were injection site reactions and constipation.
Please see accompanying Aimovig® full Prescribing Information.
Contraindication: Aimovig® is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. Reactions have included anaphylaxis and angioedema.
Hypersensitivity reactions: Hypersensitivity reactions, including rash, angioedema, and anaphylaxis, have been reported with Aimovig® in post marketing experience. Most reactions were not serious and occurred within hours of administration, although some occurred more than one week after administration. If a serious or severe
References: 1. Aimovig® (erenumab-aooe) Prescribing Information. Thousand Oaks, CA: Amgen Inc; 2021. 2. Data on file, Amgen; [AMG 334 Simulation Effectiveness]. 3. Data on file, Amgen; [TRPT-025604 AMG DDIR, August 19, 2016]. 4. Mead J, Dammerman R, Rasmussen S. Patient reported ease-of-use with a disposable autoinjector in individuals with migraine. Patient Prefer Adherence. 2020;14:1137-1144. doi:10.2147/PPA.S248584. 5. American Headache Society. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache. 2021. doi:10.1111/head.14153. 6. Data on file, Amgen; 2023.