Indication Aimovig® (erenumab-aooe) is indicated for the preventive treatment of migraine in adults.
Indication Aimovig® (erenumab-aooe) is indicated for the preventive treatment of migraine in adults.
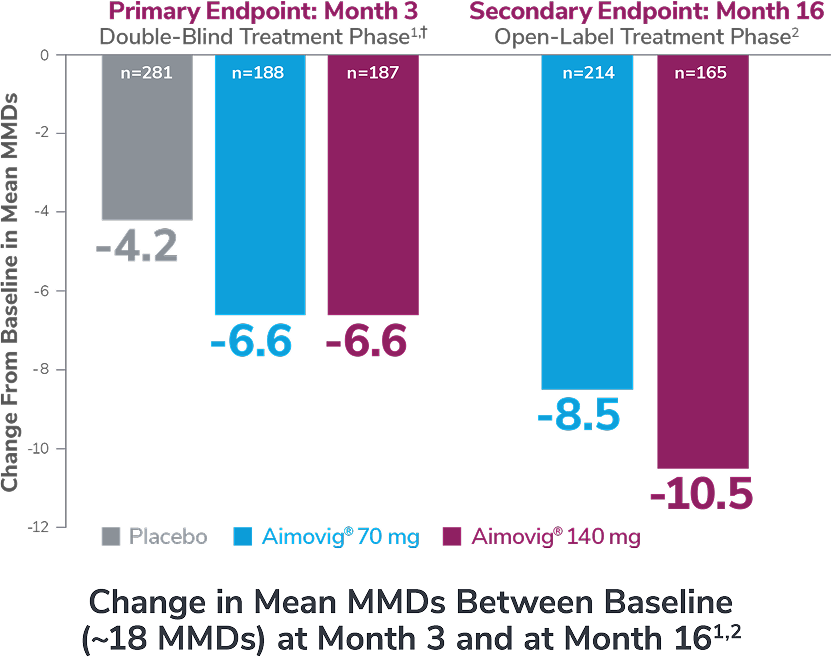
In a phase 3 EM study
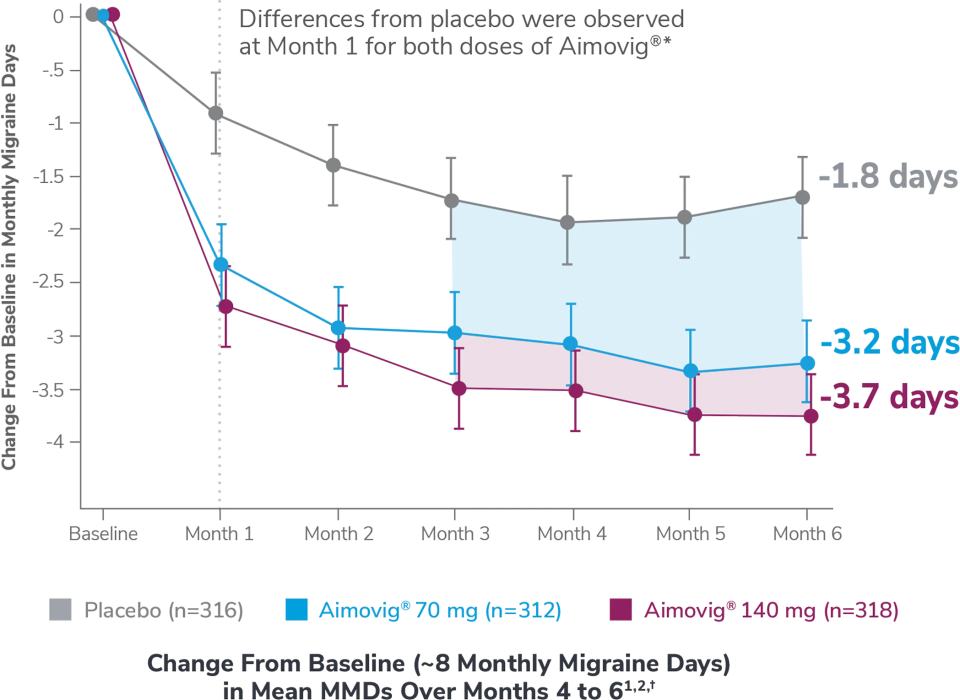
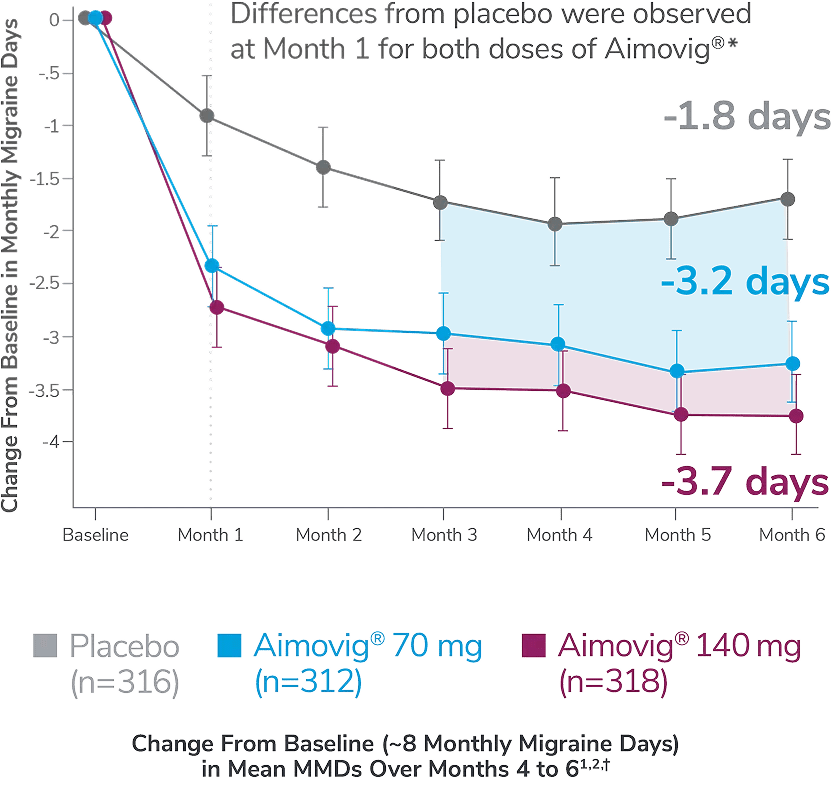
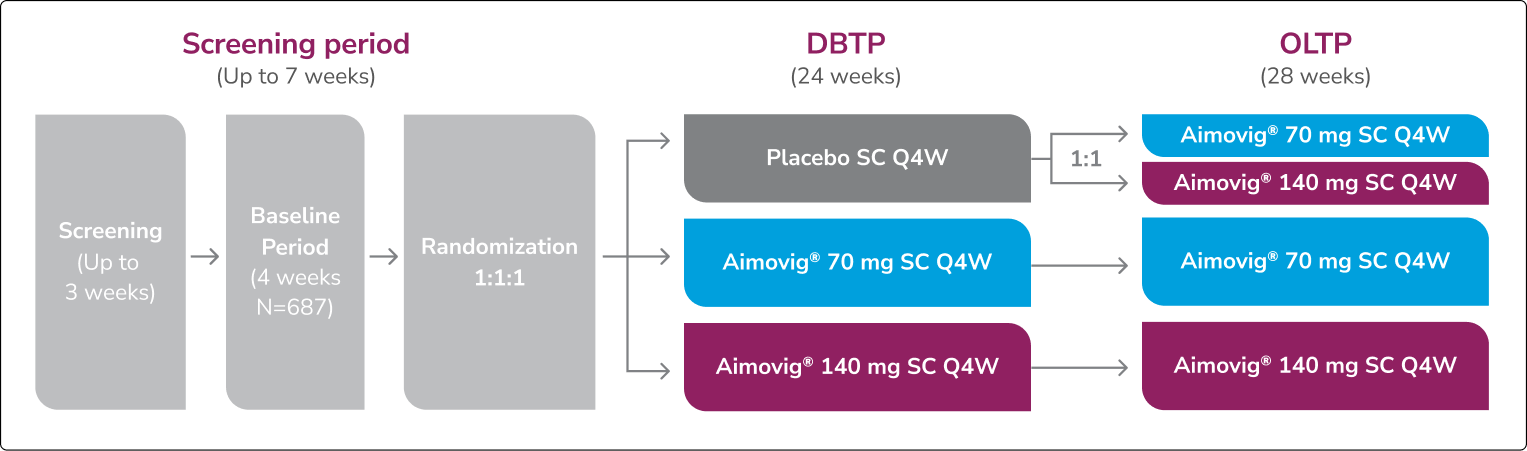
This was a phase 3, randomized double-blind, placebo-controlled study of adult episodic migraine patients.1
Aimovig® was evaluated for the prevention of episodic migraine in patients aged 18 to 65 years with 4 to 14 monthly migraine days (MMDs) in this multicenter, 24-week study.1
*Earliest post-baseline prespecified assessment.1
†Least-square means are presented. For Aimovig® 70 mg, the difference from placebo was -1.4 (95% CI: -1.9, -0.9). For Aimovig® 140 mg, the difference from placebo was -1.9 (95% CI: -2.3, -1.4).1,2
EM=episodic migraine; MMDs=monthly migraine days.


ATP=active treatment phase; DBTP=double-blind treatment phase.
In a phase 2 EM study
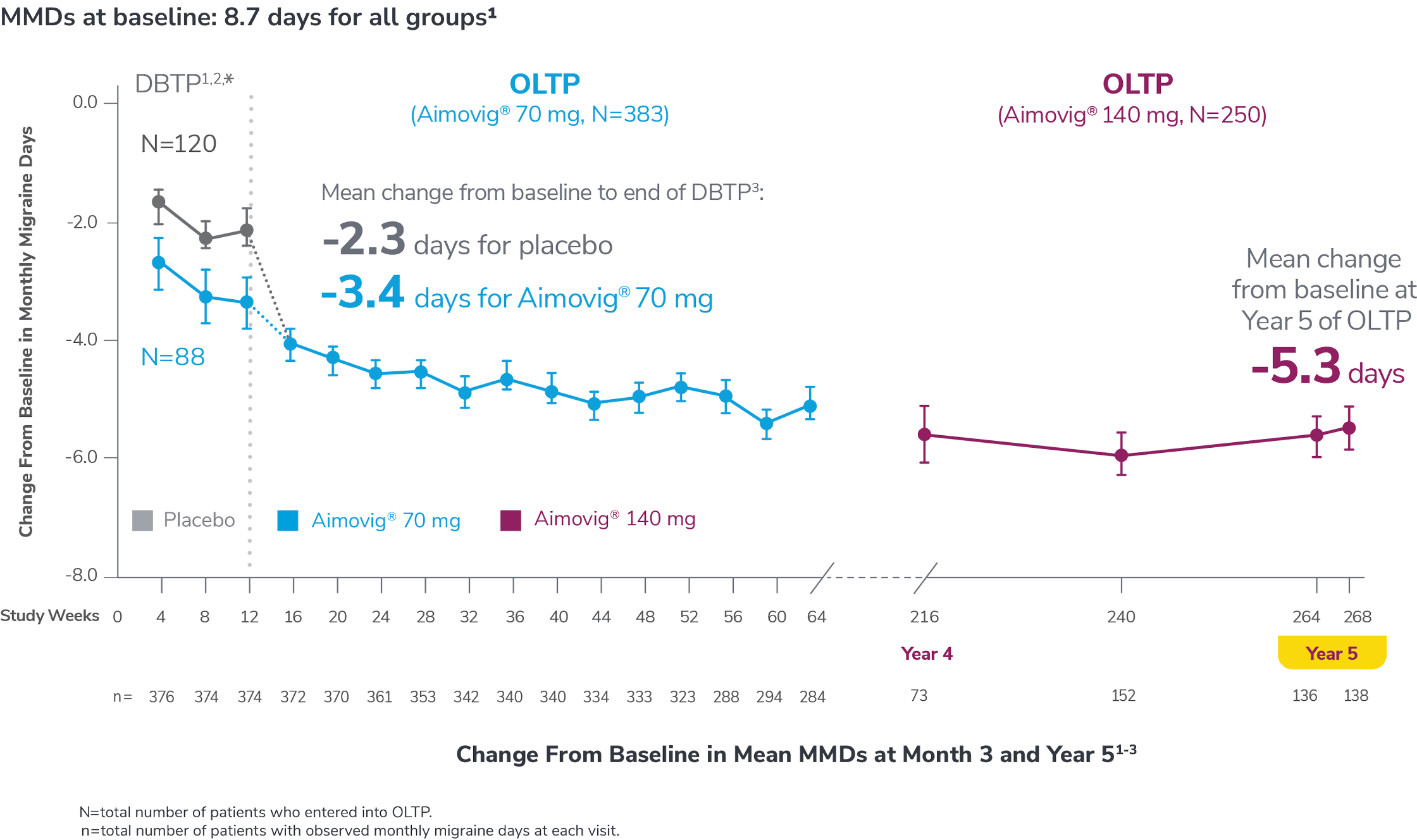
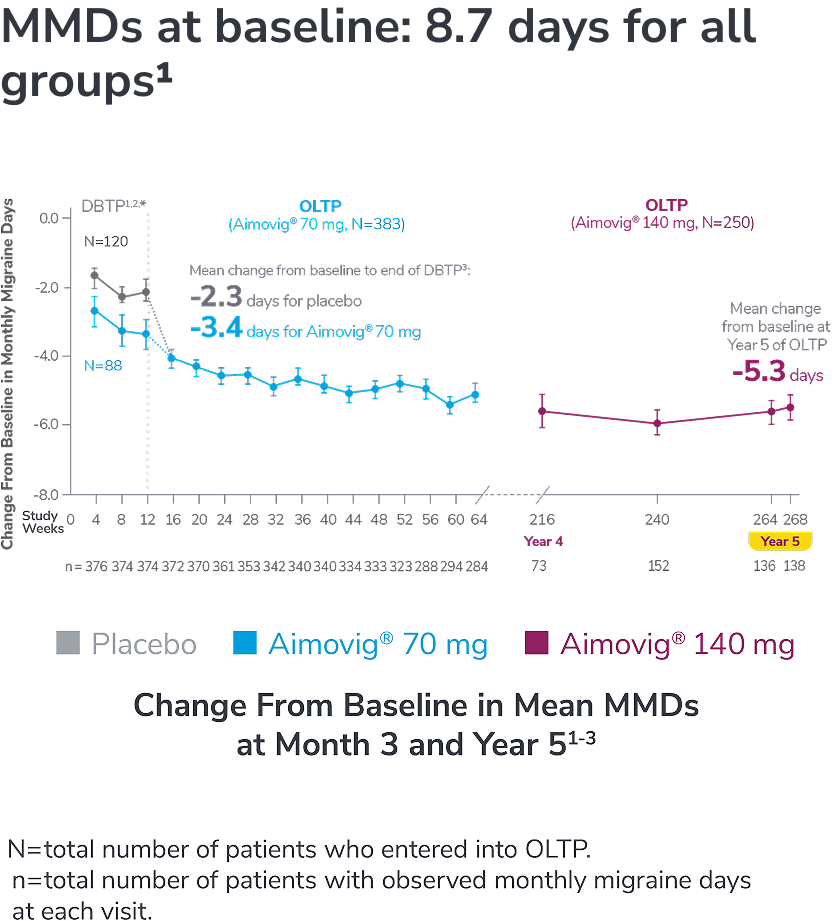
Consider open-label treatment phase study limitations when interpreting results. The OLTP was not blinded, not controlled, and included inherent self-selection bias for remaining in the trial. Overall, a total of 167 patients (43.6%) discontinued during the study, of which 18 patients (4.7%) discontinued due to adverse events.1
*Study included additional dosage arms, which were not included in the FDA-approved labeling.
DBTP=double-blind treatment phase; MMDs=monthly migraine days; OLTP=open-label treatment phase.
DBTP=double-blind treatment phase; MMDs=monthly migraine days; OLTP=open-label treatment phase.
In a phase 3 EM study
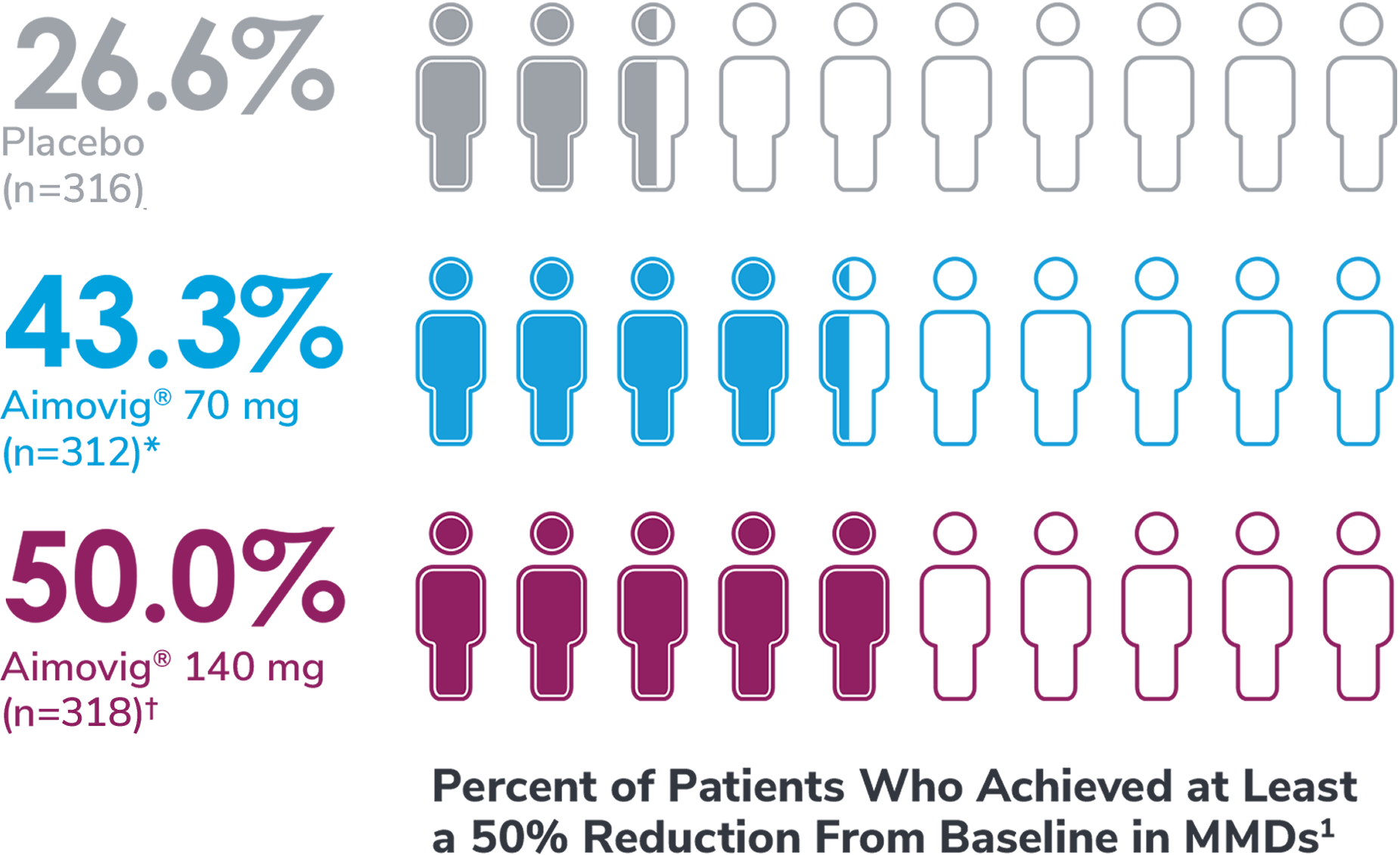
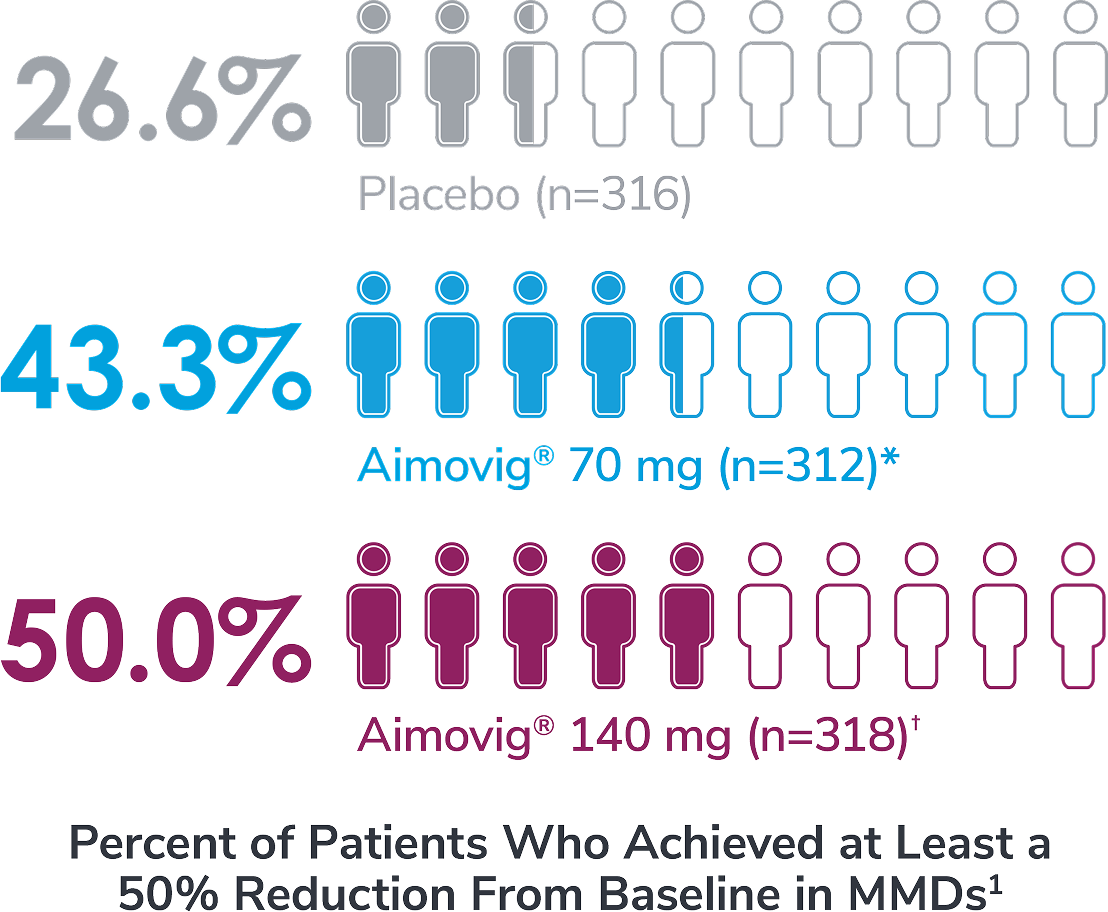
*Odds of achieving a ≥50% reduction were significantly higher (2.1x, 95% CI: 1.5, 3.0) for the Aimovig® 70 mg group than for the placebo group (P<0.001).1,2
†Odds of achieving a ≥50% reduction were significantly higher (2.8x, 95% CI: 2.0, 3.9) for the Aimovig® 140 mg group than for the placebo group (P<0.001).1,2
In a phase 2 EM study
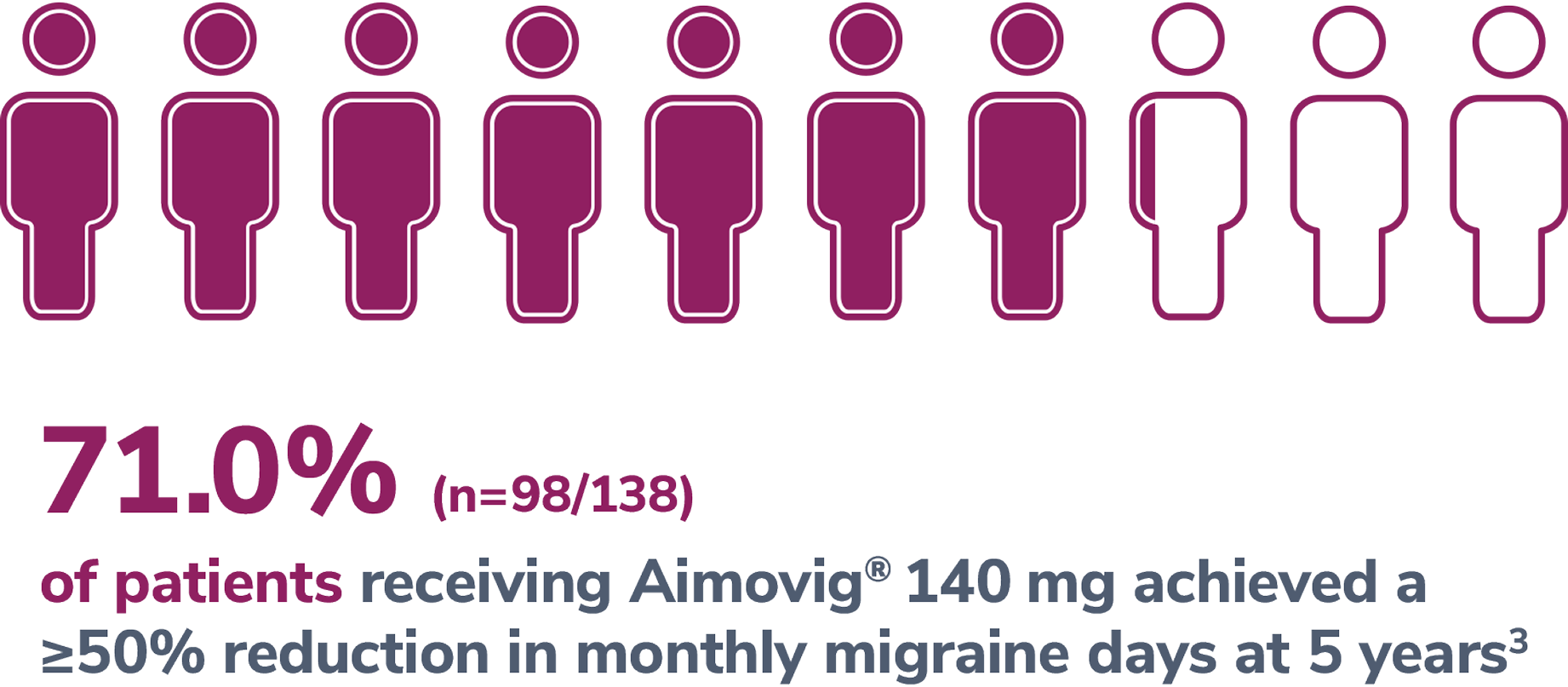
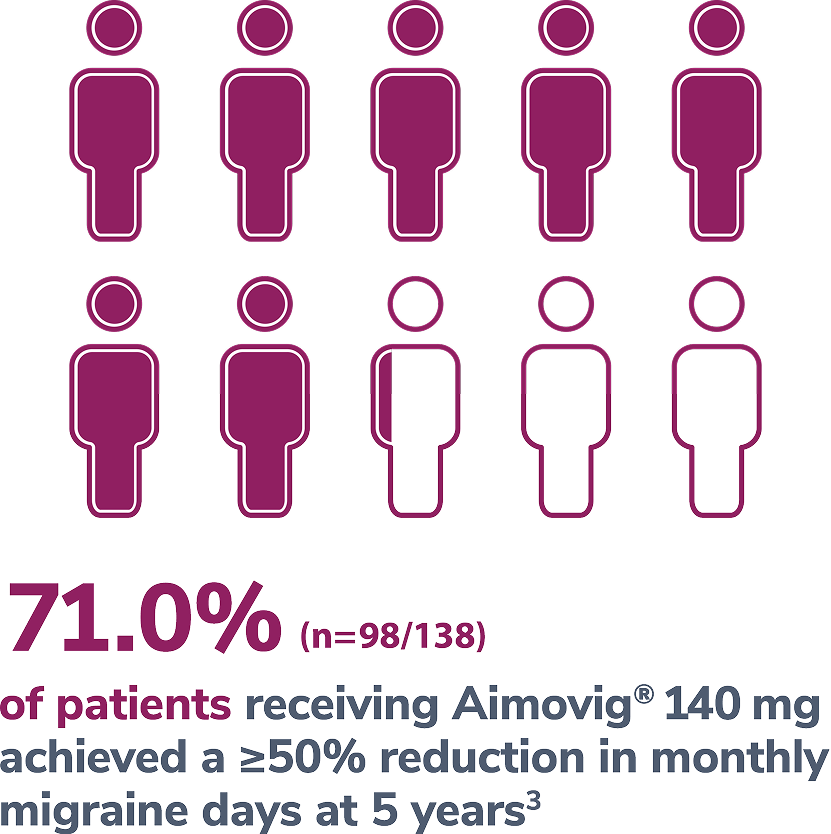
Consider open-label treatment phase study limitations when interpreting results. The OLTP was not blinded, not controlled, and included inherent self-selection bias for remaining in the trial. Overall, a total of 167 patients (43.6%) discontinued during the study, of which 18 patients (4.7%) discontinued due to adverse events.5
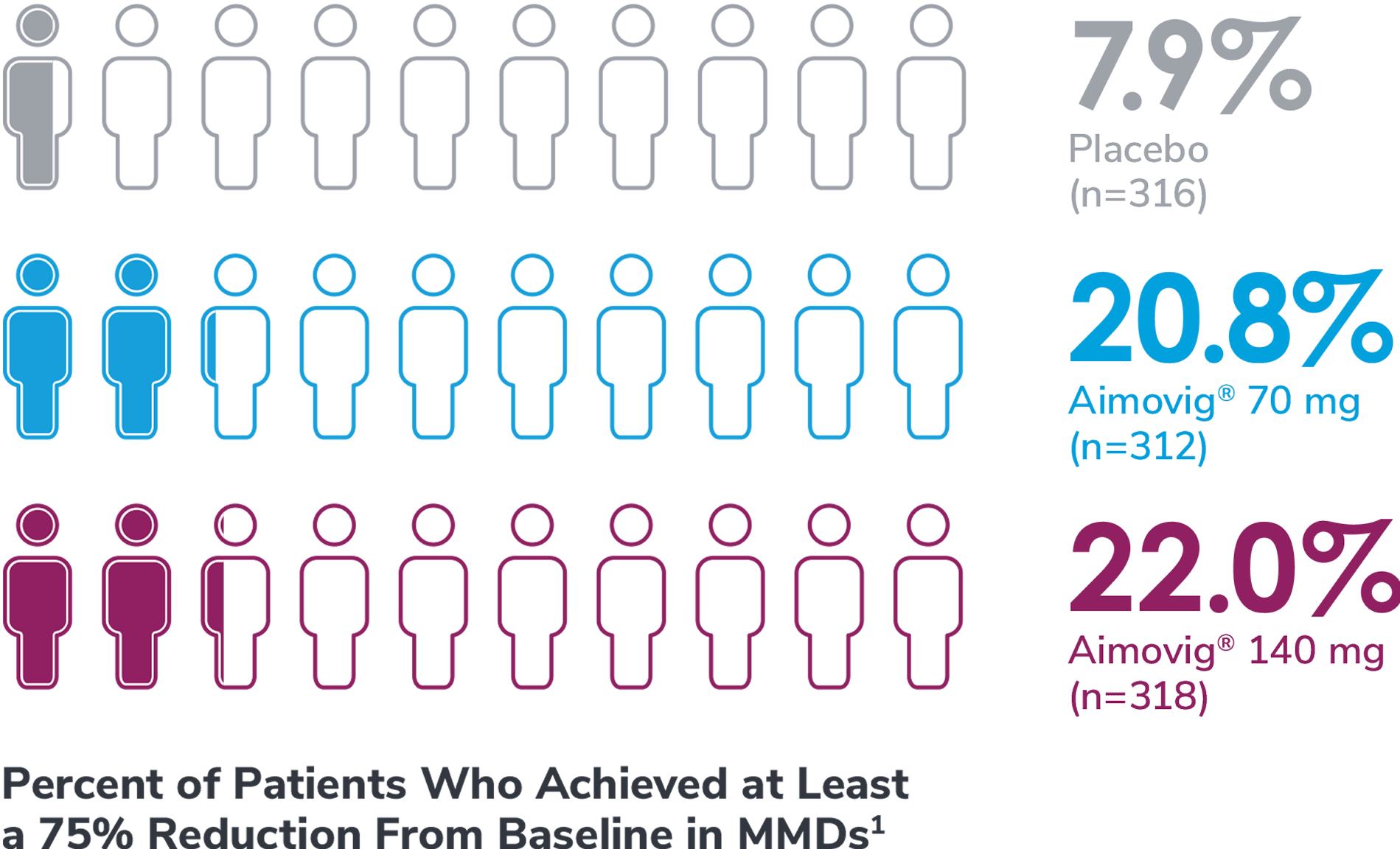
In a phase 3 EM study exploratory analysis
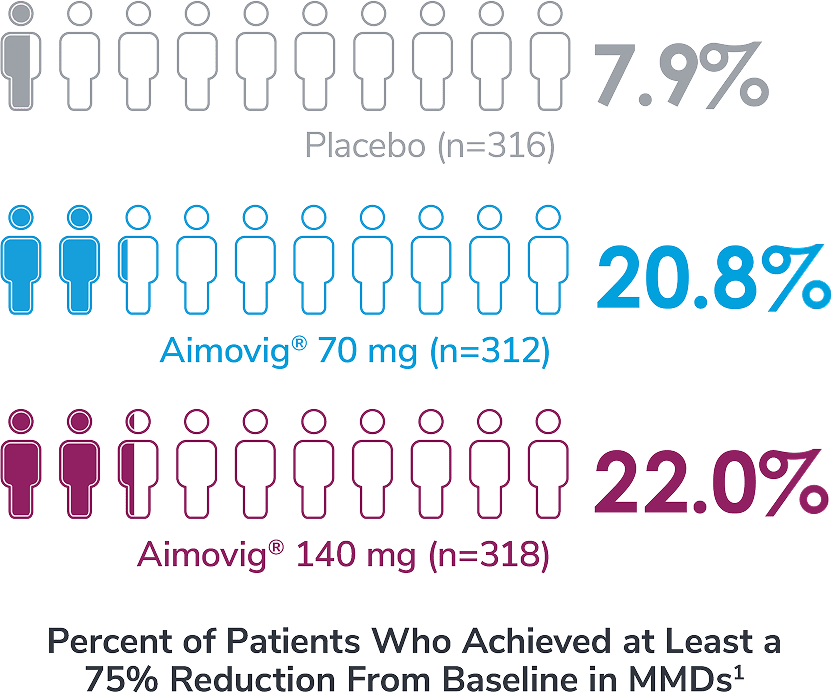
In a phase 2 EM study

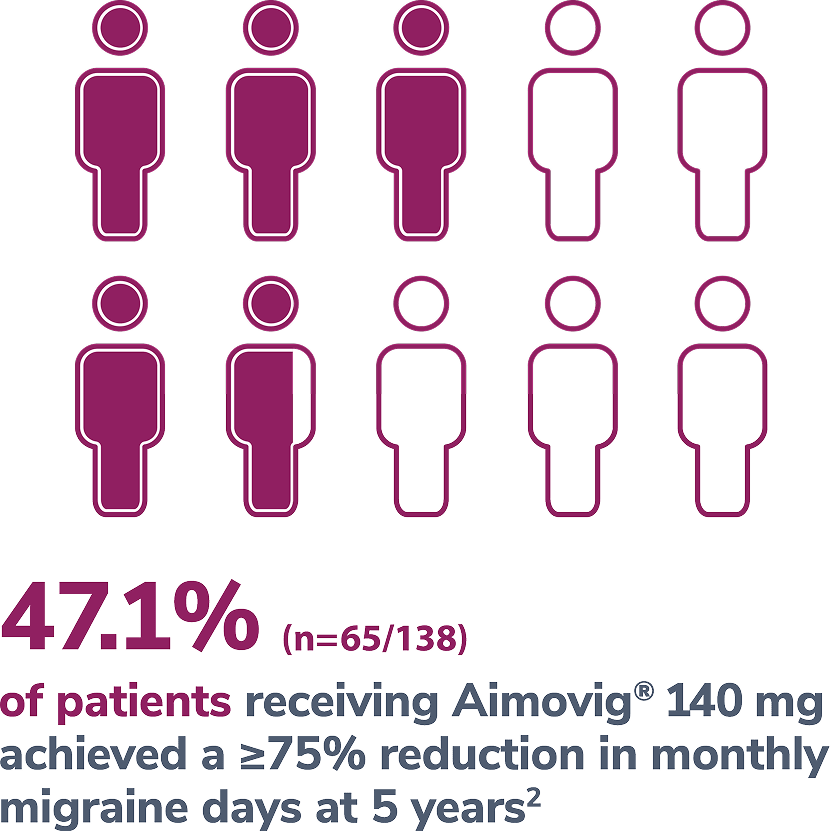
Consider open-label treatment phase study limitations when interpreting results. The OLTP was not blinded, not controlled, and included inherent self-selection bias for remaining in the trial. Overall, a total of 167 patients (43.6%) discontinued during the study, of which 18 patients (4.7%) discontinued due to adverse events.5
*Represents only patients who entered into open-label treatment phase.4
DBTP=double-blind treatment phase; EM=episodic migraine; MMDs=monthly migraine days; OLTP=open-label treatment phase.
In a phase 2 CM study
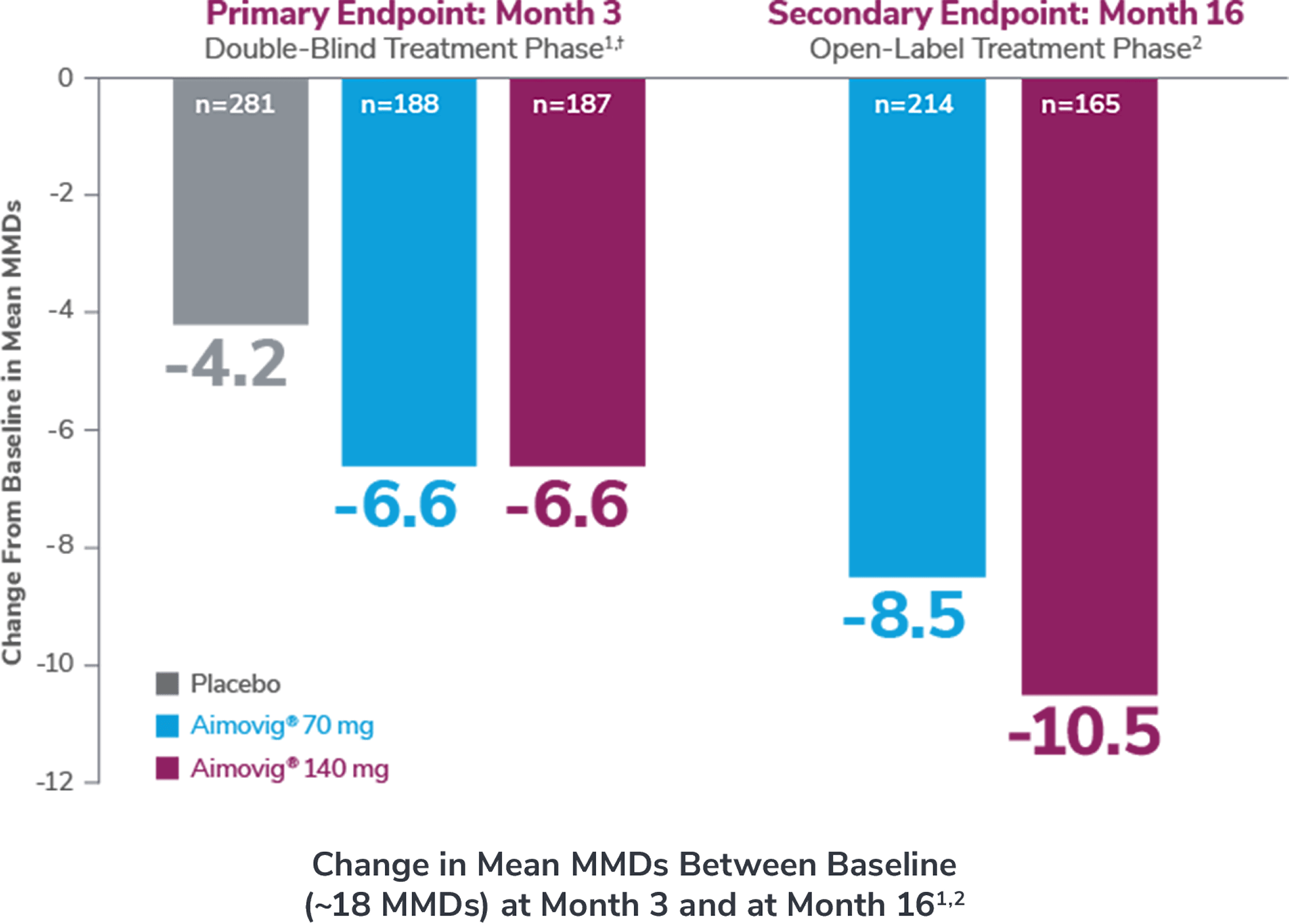
Consider open-label treatment phase study limitations when interpreting results. The OLTP was not blinded, not controlled, and included inherent self-selection bias. Overall, 22.8% (n=139) patients discontinued Aimovig® during the study, of which 2.6% (n=16) patients discontinued due to AEs.2
*Versus placebo in the DBTP; vs baseline in OLTP.1,2
†Least-square means are presented. Statistically significant reductions in monthly migraine days at Month 3 for Aimovig® 70 mg and 140 mg vs placebo (P<0.001). For both Aimovig® 70 mg and Aimovig® 140 mg, the difference from placebo was -2.5% (95% CI: -3.5, -1.4).1,3
AE=adverse event; CM=chronic migraine; DBTP=double-blind treatment phase; EM=episodic migraine; MMDs=monthly migraine days; OLTP=open-label treatment phase; QM=once a month.
Medication overuse (MOU) is the frequent use of acute treatments for an underlying headache disorder, such as chronic migraine. This overuse leads to the development of a secondary headache disorder known as medication overuse headache (MOH).4
 ≥15 headache days per month
≥15 headache days per month  >3 months of regular overuse of one or more drugs that can be taken for acute and/or symptomatic treatment of headache
>3 months of regular overuse of one or more drugs that can be taken for acute and/or symptomatic treatment of headache  Developed a new type of headache or a significant worsening of their pre-existing headache
Developed a new type of headache or a significant worsening of their pre-existing headache  Not better accounted for by another ICHD-3 diagnosis
Not better accounted for by another ICHD-3 diagnosis In a phase 4 CM with MOH study
Aimovig® was studied in a phase 4, randomized, double-blind, parallel group, placebo-controlled study, evaluating efficacy and safety in adults with chronic migraine for at least 12 months who have a history of at least one preventive treatment failure and have been diagnosed with MOH. This analysis comprised 584 patients in a non-opioid-treated cohort who were randomized 1:1:1.5
Primary endpoint5
Key secondary endpoints5





*Defined by mean monthly treatment of AHMDs <10 days over Months 4, 5, and 6 and/or mean MHDs <14 days (in a 28-day study month) over Months 4, 5, and 6 of the DBTP, where AHMDs include any eDiary day in which an acute headache medication intake is reported.
†“Absence of MOH” is achieved when mean monthly AHMDs <10 days and/or mean MHDs <14 days over the respective 3-month period.
‡Least-square means are presented. Statistically significant reductions in AHMDs for Aimovig® 140 mg vs placebo (difference from placebo -2.7 [95% Cl: -3.9, -1.6]; P<0.001). Reductions in AHMDs for Aimovig® 70 mg vs placebo were not statistically significant (difference from placebo -1.2 [95% Cl: -2.4, -0.1]).
AHMDs=acute headache medication days; CM=chronic migraine; DBTP=double-blind treatment phase; ICHD-3=International Classification of Headache Disorder, 3rd edition;
MHDs=monthly headache days; MOH=medication overuse headache; Q4W=once every 4 weeks; SC=subcutaneous.
In a phase 2 CM study subgroup analysis


Analysis is exploratory and has not been adjusted for multiple comparisons. No conclusions of statistical or clinical significance can be drawn.
*Preventive medications had failed due to lack of efficacy or intolerance by self-report.
CM=chronic migraine; MMDs=monthly migraine days.
Contraindication: Aimovig® is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. Reactions have included anaphylaxis and angioedema.
Hypersensitivity Reactions: Hypersensitivity reactions, including rash, angioedema, and anaphylaxis, have been reported with Aimovig® in post marketing experience. Most reactions were not serious and occurred within hours of administration, although some occurred more than one week after administration. If a serious or severe reaction occurs, discontinue Aimovig® and initiate appropriate therapy.
Constipation with Serious Complications: Constipation with serious complications has been reported following the use of Aimovig® in the postmarketing setting. There were cases that required hospitalization, including cases where surgery was necessary. The onset of constipation was reported after the first dose in a majority of these cases, but patients also reported later on in treatment. Aimovig® was discontinued in most reported cases. Constipation was one of the most common (up to 3%) adverse reactions reported in clinical studies.
Monitor patients treated with Aimovig® for severe constipation and manage as clinically appropriate. Concurrent use of medications associated with decreased gastrointestinal motility may increase the risk for more severe constipation and the potential for constipation-related complications.
Hypertension: Development of hypertension and worsening of pre-existing hypertension have been reported following the use of Aimovig® in the postmarketing setting. Many of the patients had pre-existing hypertension or risk factors for hypertension. There were cases requiring pharmacological treatment and, in some cases, hospitalization. Hypertension may occur at any time during treatment but was most frequently reported within seven days of dose administration. In the majority of the cases, the onset or worsening of hypertension was reported after the first dose. Aimovig® was discontinued in many of the reported cases.
Monitor patients treated with Aimovig® for new-onset hypertension, or worsening of pre-existing hypertension, and consider whether discontinuation of Aimovig® is warranted if evaluation fails to establish an alternative etiology.
Raynaud's Phenomenon: Development of Raynaud’s phenomenon and recurrence or worsening of preexisting Raynaud’s phenomenon have been reported in the postmarketing setting following the use of CGRP antagonists, including AIMOVIG. Many of the cases reported serious outcomes, including hospitalizations and disability, generally related to debilitating pain.
AIMOVIG should be discontinued if signs or symptoms of Raynaud’s phenomenon develop, and patients should be evaluated by a healthcare provider if symptoms do not resolve. Patients with a history of Raynaud’s phenomenon should be monitored for, and informed about the possibility of, worsening or recurrence of signs and symptoms.
Adverse Reactions: The most common adverse reactions in clinical studies (≥ 3% of Aimovig®-treated patients and more often than placebo) were injection site reactions and constipation.
Aimovig® (erenumab-aooe) is indicated for the preventive treatment of migraine in adults.
Please see Aimovig® full Prescribing Information.
Contraindication: Aimovig® is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. Reactions have included anaphylaxis and angioedema.
Hypersensitivity reactions: Hypersensitivity reactions, including rash, angioedema, and anaphylaxis, have been reported with Aimovig® in post marketing experience. Most reactions were not serious and occurred within hours of administration, although some occurred more than one week after administration. If a serious or severe
References: 1. Aimovig. Package insert. Amgen Inc; 2025. 2. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132. 3. Goadsby PJ, Reuter U, Hallström Y, et al. One-year sustained efficacy of erenumab for episodic migraine: Results of the STRIVE study. Neurology. 2020;95:e469-e479. doi:10.1212/WNL.0000000000010019.
References: 1. Ashina M, Goadsby PJ, Reuter U, et al. Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28:1716-1725. doi:10.1111/ene.14175. 2. Data on file, Amgen; [Ph 2 EM CSR T14-1.1, January 28, 2016]. 3. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382-390.
References: 1. Aimovig. Package insert. Amgen Inc; 2025. 2. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132. 3. Data on file, Amgen; [Ph 2 EM CSR, November 25, 2020]. 4. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382-390. 5. Ashina M, Goadsby PJ, Reuter U, et al. Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28:1716-1725. doi:10.1111/ene.14175.
References: 1. Bröessner G, Reuter U, Bonner J, et al. Achievement of ≥75% and 100% response in patients treated with erenumab: 24-week results from the STRIVE study. Poster presented at: 18th Congress of the International Headache Society; September 7-10, 2017; Vancouver, Canada. 2. Data on file, Amgen; [Ph 2 EM CSR, November 25, 2020]. 3. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382-390. 4. Data on file, Amgen; [Ph 2 EM CSR T14-4.3.19, January 28, 2016]. 5. Ashina M, Goadsby PJ, Reuter U, et al. Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28:1716-1725. doi:10.1111/ene.14715.
References: 1. Aimovig. Package insert. Amgen Inc; 2025. 2. Tepper SJ, Ashina M, Reuter U, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: Results from a 52-week, open-label extension study. Cephalalgia. 2020;40(6):543-553. 3. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434. 4. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211. 5. Tepper SJ, Dodick D, Lanteri-Minet M, et al. A Phase 4 Randomized Placebo-Controlled Study to Evaluate the Efficacy and Safety of Erenumab in Adults with Medication Overuse Headache. Poster presented at: American Headache Society 65th Annual Scientific Meeting; June 15-18, 2023; Austin, TX.
Reference: 1. Ashina M, Tepper SJ, Brandes JL, et al. Efficacy and safety of erenumab (AMG 334) in chronic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2018;38(10):1611-1621.